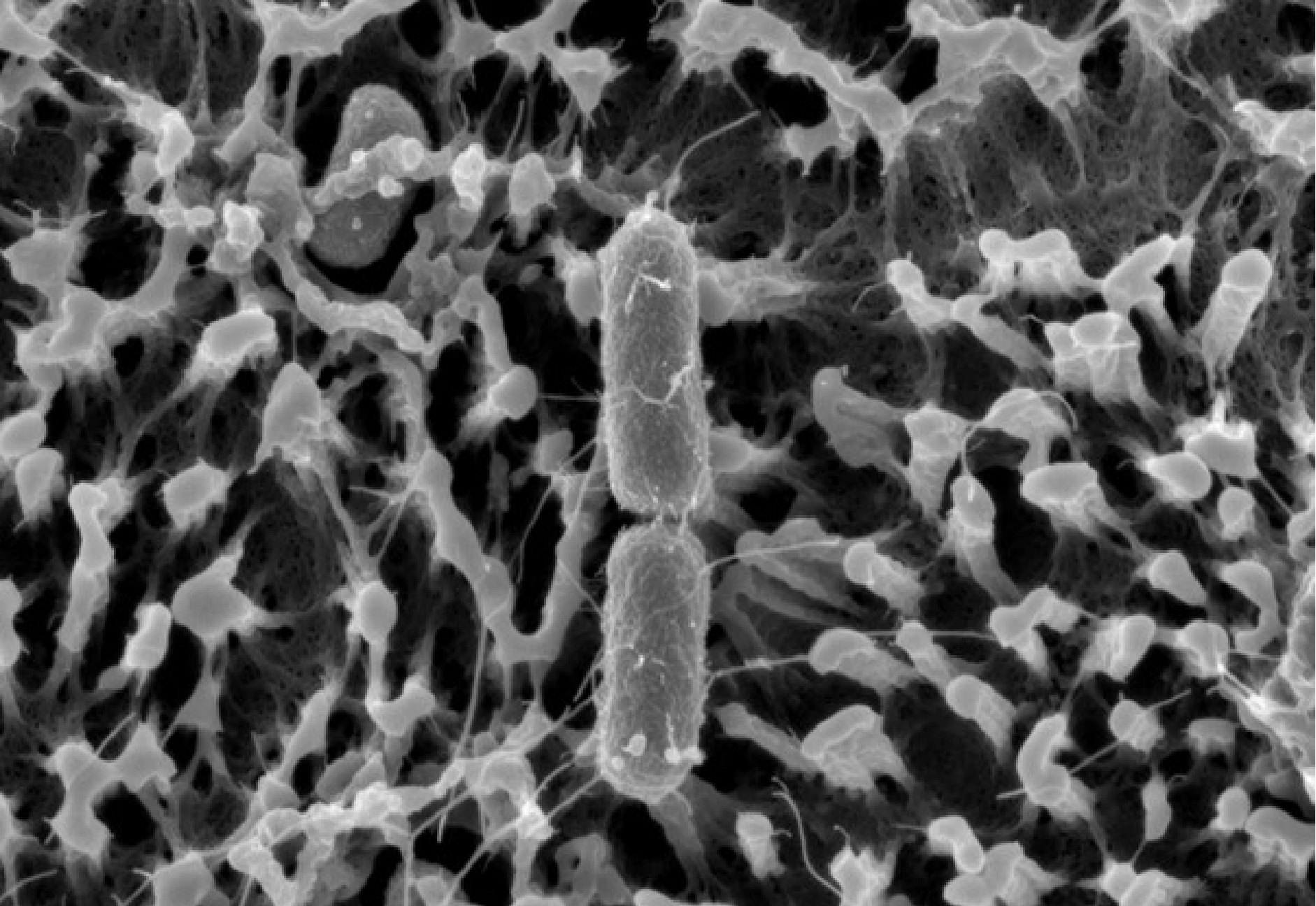
Scanning Electron Microscopy (SEM) image of Salmonella attached to microvilli of a 2Dpolarized intestinal epithelial monolayer. Imaging performed by Nora Trinks in collaboration with Sara Henriksson at Umeå Center for Electron Microscopy (UCEM).
Hello, I´m Finja Dienhart, a PhD candidate with Mikael Sellins’ laboratory at Uppsala University. I grew up in Münster, Germany, where I also studied for my bachelor’s and master’s at the University of Münster. During my bachelor’s studies in molecular bioscience, I discovered my passion for cell and infection biology. Thus, I decided to pursue this path with a master’s in molecular biomedicine. During my studies, it became evident that I wished to deepen my knowledge and skillset by pursuing a PhD.
In doing so, I have joined the Sellin Lab, where we investigate bacterium–host cell interplay in the intestine. The intestinal epithelium functions as a vital defense barrier against the external environment in order to avoid infection and inflammation. This barrier function is achieved by the specialized apical surface on intestinal epithelial cells (IECs). The cells’ microvilli are covered with a thick “forest” of highly glycosylated transmembrane proteins, forming the so-called glycocalyx. On top of this lies a secreted layer of soluble mucus, adding to the barrier function. But certain intestinal pathogens are equipped to overcome this barrier and to use IECs as a replicative niche.
Traditionally, the bacterial–host cell interplay has been studied in tumor cell line models, leading to numerous insights about infection mechanisms. However, classic cell culture models poorly recapitulate the described physiological features and architecture of the intact human intestinal tract. This is why we combine organoid technology, which captures key aspects of mammalian gut tissue, with bacterial genetics and high-resolution live imaging at the Sellin Lab. We aim to uncover physiologically relevant mechanisms used by intestinal pathogens, like Salmonella and Shigella, to overcome the IEC barrier, and identify the host cell and tissue features that counter this onslaught.
By using organoid technology, we, in ongoing work, have shown that the maturation status of the gut epithelium determines bacterial success or failure during invasion. An “immature” intestinal epithelium is much more prone to invasion compared to a “mature” epithelium consisting of specialized absorptive cells (enterocytes or colonocytes). As these studies were performed without taking the full cell type diversity into account, we now want to increase the complexity of our model by, for example, including the soluble mucus layer, which is found in vivo on top of the epithelium.
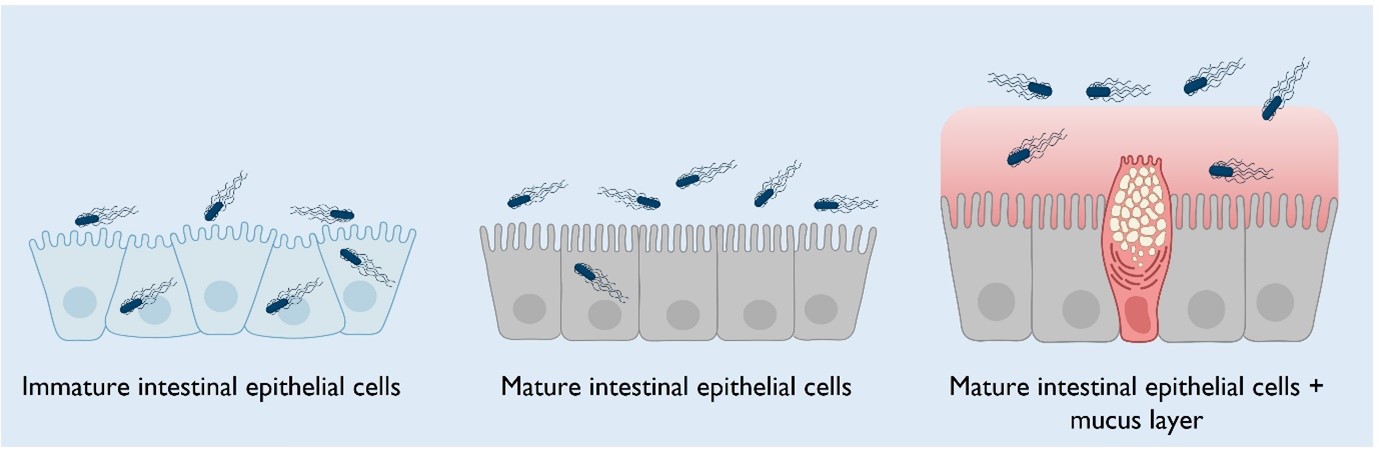
In my PhD project, I plan to further increase the maturation status of our 2D organoid-derived IEC system, aiming to mimic the epithelial cell barrier across gut segments, either with or without a protective mucus layer on top. By refining our model system, we will decipher how specific epithelial cell surface features influence the frequency and the outcome of bacterial invasion, helping us to understand gut disease mechanisms.
